(c) The above two changes result in a higher frequency of effective collision, and consequently a higher rate of reaction. Example: Two sets of experiments are carried out as shown below. Set X: 2 cm of magnesium ribbon is added to 50 cm 3 of 1 mol dm -3 sulphuric acid at room temperature.Q. A chemical reaction occurs involving alka seltzer and water takes place at 25 °C. If the temperature is increased to 75 °C how do the number of effective collisions and the rate of the reaction compare to the original reaction?Number of collisions per unit time increases. Number of effective collisions per unit time increases. Rate of reaction increases. Effect Of Catalyst On Rate Of Chemical Reactions. A catalyst is a chemical substance that changes the rate of reaction without itself undergoing any permanent chemical change at the end of the reaction.This method is very effective, especially when a limited number of temperature-dependent rate constants are available for the reaction of interest. Exercise \(\PageIndex{1}\) The rate constant for the rate of decomposition of N 2 O 5 to NO and O 2 in the gas phase is 1.66 L/mol/s at 650 K and 7.39 L/mol/s at 700 K:Reaction kinetics is the study of the rate of chemical reactions, and reaction rates can vary greatly over a large range of time scales. Some reactions can proceed at explosively fast rates like the detonation of fireworks (Figure 17.1 " Fireworks at Night Over River"), while others can occur at a sluggish rate over many years like the rusting of barbed wire exposed to the elements (Figure
Collision Theory | Chemical Reactions Quiz - Quizizz
The correct option is this: DECREASING THE TEMPERATURE. During the process of chemical reaction, the reacting molecules typically move about randomly in the reacting vessels colliding with one another and with the wall of the container. The rate of movement of the reacting particles can be reduced by decreasing the temperature of the reaction.In a chemical reaction, collisions between the molecules provide the potential energy needed to break the bond. The effective collisions must occur with certain minimum amounts of energy . In a large sample, the greater the number of effective collisions, and the faster the rate of reaction. All of the above. Tags: Question 27 . SURVEY .A chemical reaction occurs as a result of collisions between reacting molecules. Therefore, the reaction rate is given by the total number of effective collisions.More is the no. of effective collisions, more will be reaction rate.Favorite Answer The factors that affect the rate of collisions in a reaction are: Effect of the nature of reactants. Effect of concentrations of reactants.

Factors Affecting Rate Of Chemical Reaction | Mini
As the number of effective collisions between reacting particles increases, the rate of reaction increases. In order for a chemical reaction to occur and products to be formed, the elements that make up those products must physically come in contact with each other. The more the constituent particles are colliding, the faster product is forming.An increase in concentration increases the rate of reaction because it increases the number of collisions. Therefore there is a greater probability of successful collisions, resulting in a chemical reaction.Just like many other chemical reactions, the rate of an enzyme-catalyzed reaction will decrease with temperature because of the decrease in the number of high energy collisions between particles.Which change will decrease the number of effective collisions during a chemical reaction? A) adding a catalyst . B) increasing the surface area . C) decreasing the temperature . D) increasing the reactant concentrations . E) increasing the volume of the reactants. Answer Save. 3 Answers.Lowering the temperature could also be used to decrease the number of collisions that would occur and lowering the temperature would also reduce the kinetic energy available for activation energy. If the particles have insufficient activation energy, the collisions will result in rebound rather than reaction.
You do not supply a selction of alternatives, but almost speaking, lowering the temperature, increasing the quantity, and reducing the power will all decrease the number of effective colisions in the gasoline phase, whiletemperature by myself will have the same effec in the liquid section.
Collision Theory For Rates of Chemical Reactions | Mini ...

Cayman Eco - Beyond Cayman Blackouts In Texas And ...

Endothermic Reaction Coordinate Diagram

Catalysis - Chemistry

Reaction Enthalpy: Concepts, Calculation Methods, Videos ...

Ch. 5: Chemical Kinetics at Bowling Green State University ...

Cayman Eco - Beyond Cayman Blackouts In Texas And ...

B2 An activated complex has A low potential energy and is ...

What is the Carbon Cycle? What is the science behind it ...

Cayman Eco - Beyond Cayman Blackouts In Texas And ...

Cayman Eco - Beyond Cayman Blackouts In Texas And ...

Cayman Eco - Beyond Cayman Blackouts In Texas And ...

PPT - Reaction Rates and Equilibrium PowerPoint ...
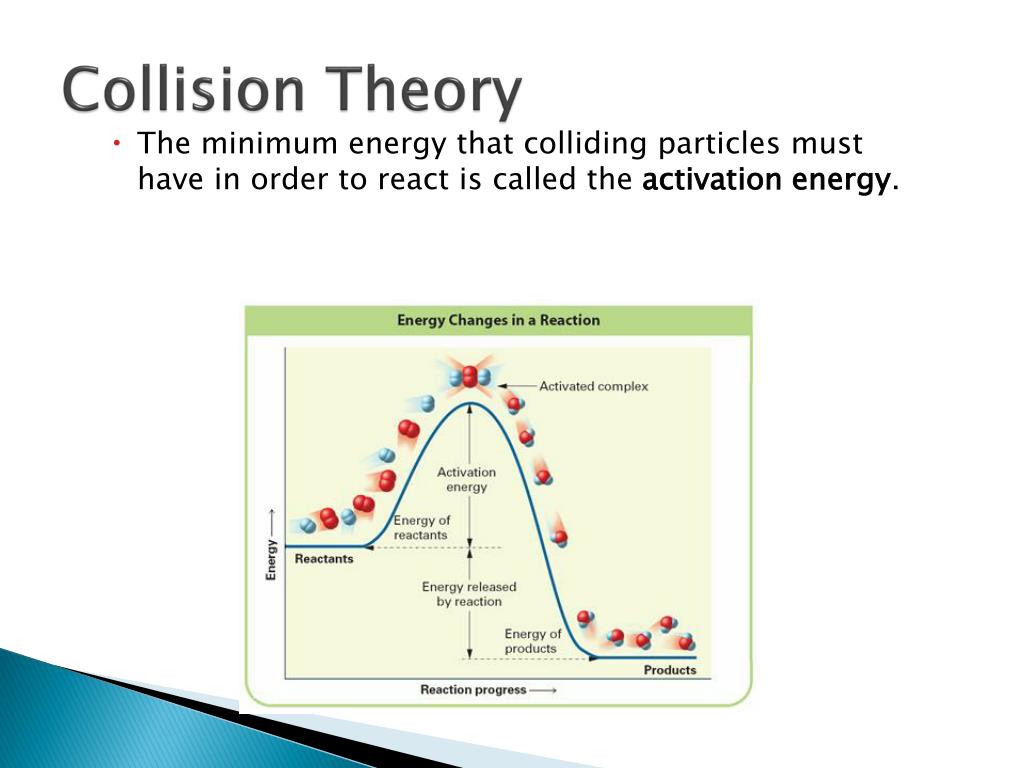
Chemical-Kinetics.pptx - Chemical Kinetics Reaction Rates ...

Uncovering the science behind reaction rates in chemical ...

PPT - Reaction Rates and Equilibrium PowerPoint ...
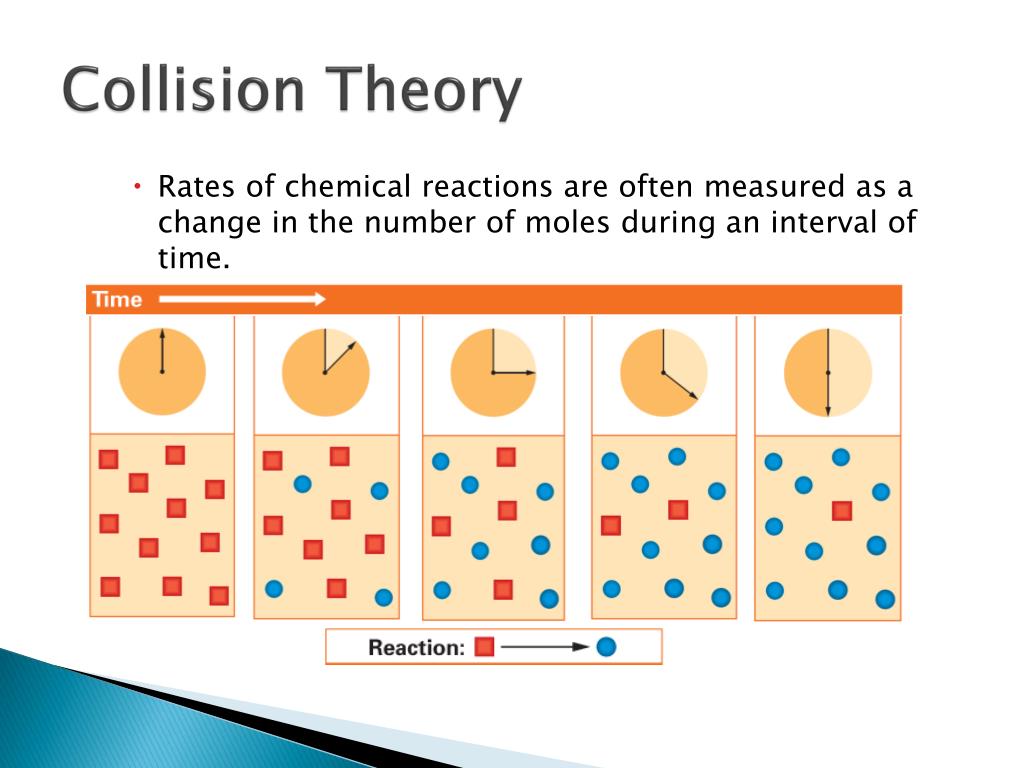
4.docx - For the reaction A 2B C the rate law is What are ...

CH103 - Chapter 8: Homeostasis and Cellular Function ...

Kinetics Notebook. | Aoxales12's Blog

Cayman Eco - Beyond Cayman Blackouts In Texas And ...

Arrhenius & Catalysts

0 Comment to "Collision Theory | Chemical Reactions Quiz - Quizizz"
Post a Comment