molecular weight of (NH4)2O or mol The SI base unit for amount of substance is the mole. 1 grams (NH4)2O is equal to 0.019202585743386 mole. Note that rounding errors may occur, so always check the results.Oxygen is O2- Electronic configuration of oxygen is 2.6, and that's how the symbol for Oxygen becomes O 2- as it requires 2 more electrons to fill up the second shell. Need to duplicate another NH4+ to balance the charge. As such the symbol is for ammonium oxide is (NH4)2OYou will first need to calculate the moles of carbon dioxide. Then write a balanced equation and use the mole ratio between carbon dioxide and ammonium carbonate ((NH4)2CO3) to determine the moles of (NH4)2CO3 required. Then calculate the mass of (NH4)2CO3 required from moles and molar mass. Convert 9.52 g of CO2 to moles.The compounds [NH4]12[(MoO2)2O(HPO4)2]4[PO4]X, X = Cl, Br are constructed from molybdenophosphate layers of unique topology that are penetrated by channels containing perfectly orderedWhen ammonium sulfate dissociates in water, NH4+ is the cation and SO4^2- is the anion. 3 0. Varaprasad. Lv 4. 8 years ago. NH4^+ CATION SO4^2- ANION. 0 0. Anonymous. 8 years ago. for every ammonium ion u need one sulfate ion. and for every sulfate ion u need 2 ammonium ions.
What is the chemical formula for ammonium oxide? - Quora
Common Cations and Anions Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3+ +3 magnesium Mg 2+ +2 carbonate CO 3 2- -2 ammonium NH 4 + +1 manganese (II) Mn 2+ +2 chlorate ClO 3 - -1 barium Ba 2+ +2 manganese (III) Mn 3+ +3 chloride Cl - -1 cadmium Cd 2+ +2 mercury (I)This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.Can you determine the products of each reaction? Learn with flashcards, games, and more — for free.In this video we'll write the correct formula for Ammonium oxide, (NH4)2O.To write the formula for Ammonium oxide we'll use the Periodic Table, a Common Ion

How many grams of ammonium carbonate are needed to
Top contributors to the provenance of Δ f H° of [NH4]+ (aq) The 16 contributors listed below account for 90.6% of the provenance of Δ f H° of [NH4]+ (aq). Please note: The list is limited to 20 most important contributors or, if less, a number sufficient to account for 90% of the provenance.The ammonium ion that results from this neutralization has its own equilibrium (Ka): NH4+(aq) + H2O(l) ⇌ H3O+(aq) + NH3(aq) *this makes the solution slightly more acidic. Acid-Base Indicators. The equivalence point in a titration can be estimated with the use of an acid-base indicator.›› (NH4)2O molecular weight. Molar mass of (NH4)2O = 52.07632 g/mol. Convert grams (NH4)2O to moles or moles (NH4)2O to grams. Molecular weight calculation: (14.0067 + 1.00794*4)*2 + 15.9994 ›› Percent composition by elementThe compound is ammonium oxide. and O2- ions are present.Koktaite ((NH4)2Ca(SO4)2.H2O) | CaH12N2O9S2 | CID 6337632 - structure, chemical names, physical and chemical properties, classification, patents, literature
Molar mass of (NH4)2O is 52.0763 g/mol Convert between (NH4)2O weight and molesElemental composition of (NH4)2OElementSymbolAtomic weightAtomsMass percentOxygenO15.9994130.7230NitrogenN14.0067253.7930HydrogenH1.00794815.4840Mass p.c compositionAtomic % compositionFormula in Hill device is H8N2O Computing molar mass (molar weight)To calculate molar mass of a chemical compound input its method and click 'Compute'. In chemical formula you might use: Any chemical part. Capitalize the primary letter in chemical symbol and use decrease case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, Ok, Cl, Al. Functional teams: D, Ph, Me, Et, Bu, AcAc, For, Ts, Tos, Bz, TMS, tBu, Bzl, Bn, Dmg parantesis () or brackets []. Common compound names. Examples of molar mass computations: NaCl, Ca(OH)2, K4[Fe(CN)6], CuSO4*5H2O, water, nitric acid, potassium permanganate, ethanol, fructose.
Molar mass calculator additionally shows common compound title, Hill formula, elemental composition, mass p.c composition, atomic p.c compositions and lets in to transform from weight to collection of moles and vice versa.
Computing molecular weight (molecular mass) To calculate molecular weight of a chemical compound input it's system, specify its isotope mass number after each component in square brackets. Examples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. Definitions of molecular mass, molecular weight, molar mass and molar weight Molecular mass (molecular weight) is the mass of 1 molecule of a substance and is expressed in the unified atomic mass gadgets (u). (1 u is the same as 1/12 the mass of 1 atom of carbon-12) Molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. Weights of atoms and isotopes are from NIST article.Give us comments about your experience with Molecular Weight Calculator.
Related: Molecular weights of amino acids
molecular weights calculated as of lateNH4)HCO3 (Ammonium Carbonate)

Bonding And Naming WS 4 Supplement For Extra

A Facile One-step Synthesis Of Super-hydrophilic (NH4)0.33WO3/WS2 Composites: A Highly Efficient Adsorbent For Methylene Blue - New Journal Of Chemistry (RSC Publishing)

Ammonium Pentaborate
Processes | Free Full-Text | Fabrication Of Ultrathin MoS2 Nanosheets And Application On Adsorption Of Organic Pollutants And Heavy Metals | HTML

Effect Of The Variation Of The Feed Time Of The Biological Reactor... | Download Scientific Diagram

Godovikovite (NH4)(Al, Fe3+)(SO4)2
Ionic Compound Names And Formulas Why Is Sodium Acetate My Favorite Compound? - Ppt Download

Study Of Thermal Decomposition Of Ammonium Paratungstate
The Thermal Decomposition Of Ammonium Polymolybdates. I
Mechanochemistry Of Fluoride Solids: From Mechanical Activation To Mechanically Stimulated Synthesis | SpringerLink

Solved: Select All Of The Reactions Below That Represent A... | Chegg.com

Writing Formulas
Struvite (NH4)Mg(PO4)• 6H2O
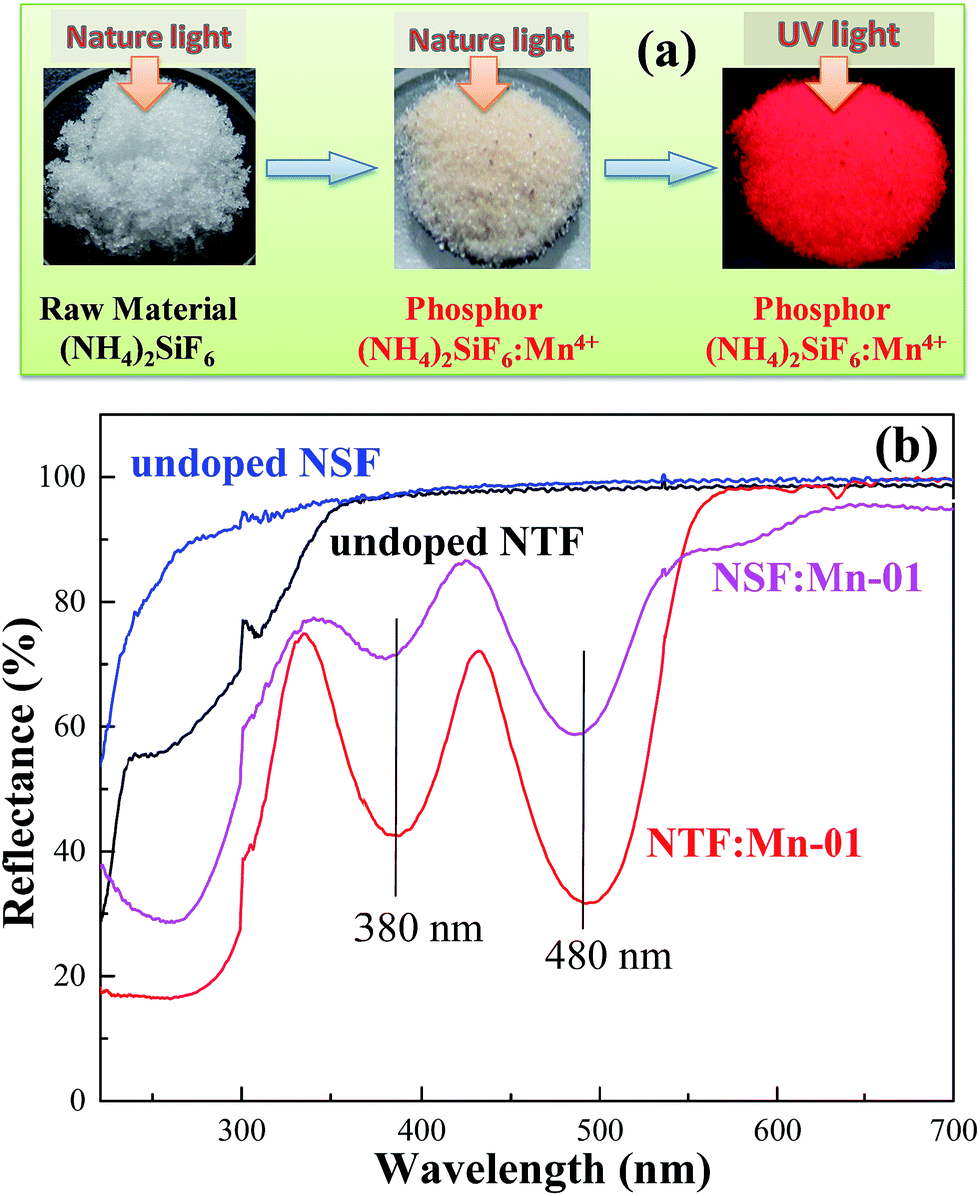
Mn4+ Doped (NH4)2TiF6 And (NH4)2SiF6 Micro-crystal Phosphors: Synthesis Through Ion Exchange At Room Temperature And Their Photoluminescence Properties - RSC Advances (RSC Publishing)
Chapter 7 Study Guide Answers

Solved: Period: Unit: Stoichiometry WS 6 - "Test Review" S... | Chegg.com

Untitled
PS1 CHE213 | Sets Of Chemical Elements | Atoms

0 Comment to "Quia - Ternary Ionic Compounds"
Post a Comment