Lewis dot structures help predict molecular geometry. This example problem shows the steps to draw a structure where an atom violates the octet rule. "Lewis Structures and the Octet Rule. An automatic procedure for writing canonical forms." J. Chem.A simple procedure for writing Lewis Electron Structures was given in a previous article entitled "Lewis Structures and the Octet Rule". Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).Drawing the Lewis Structure for NO+. Viewing Notes There are a total of 10 valence electrons in NO+. Be sure to put brackets and a positive sign around the NO+ Lewis structure to show that it is an ion.Lewis structure of NO3- ion is drawn step by step in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge are considered to draw the You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future.Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
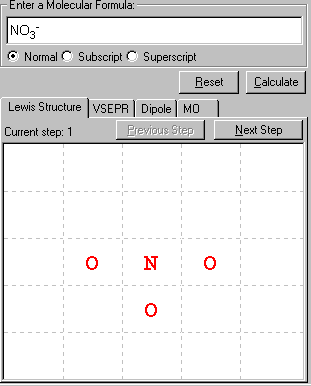
Method for Lewis dot structures: The nitrate ion lewis structures
For the BrF3 Lewis structure, calculate the total number of valence electrons for the BrF3... Try to draw the BrF3 Lewis structure before watching the video. Try structures similar to BF3 for more practice.O=N^(+)(-O^-)_2 is the standard Lewis structure. Going from left to right, there are 6 electrons around O (hence neutral), 4 electrons around N (hence positive), and 7 electrons around the rightmost oxygens (hence each is a formal anion). The formal charge can be summed to -1 as required.chemistry questions and answers. What Is The Lewis Structure Of NO3+.Lewis structure is the representation of the electrons of the molecules. There are lone pairs and valence electrons which help in determining the Even when we draw, it's Lewis structure we do not see any dipole moment or the polar bonds in it as the overall charge itself is negative on the ion.
NO+ Lewis Structure - How to draw the Lewis Dot Structure for NO+
But why is structure number one preferred over structure number two? Why does carbon prefer to have a triple bond with nitrogen, rather than have a Do the same exercise for structure #2 and you find that the negative charge is on nitrogen. Since oxygen is more electronegative then nitrogen, the...NO3- Lewis Structure: How to Draw the Lewis Structure for NO3 A step-by-step explanation of how to draw the NO3- Lewis Structure (Nitrate Ion). Get more chemistry help at www.Breslyn.org.I quickly take you through how to draw the Lewis Structure of NO3- (Nitrate Ion). I also go over the resonance, hybridization, shape and bond angle.Lewis Structure. Main Group Halides. Beryllium Fluoride. BeF 2. Lewis Structure. Lewis Structure. Phosphorus Pentachloride. PCl 5. Lewis Structure. Nitrogen Dioxide. NO 2.Lewis Structure - Free download as Powerpoint Presentation (.ppt), PDF File (.pdf), Text File (.txt) or view presentation slides online. When they form ions, transition metals lose valence electrons first and d-electrons second (if at all). Rh. The electron configuration of Fe 3+ is which of the following?
Lewis structure of NO3- ion is drawn step by step on this tutorial. Total valence electrons of nitrogen and oxygen atoms and unfavorable rate are considered to attract the NO3- lewis structure. You will each and every reality of drawing lewis buildings from this tutorial which will assist you to to draw more lewis buildings sooner or later.
Lewis Structure of nitrite ion
Now, we are going to learn, how to attract this lewis structure of NO3- ion.
Steps of drawing NO3- lewis structure
Following steps are required to attract NO3- lewis structure and they're explained in detail in this instructional.
Find general collection of electrons of the valance shells of nitrogen and oxygen atoms and together with rate of the anion Total electrons pairs in valence shells Center atom selection from nitrogen and oxygen atom Put lone pairs on atoms Stability of lewis structure - Check the stability and minimize fees on atoms by way of converting lone pairs to bonds.Drawing correct lewis structure is essential to draw resonance constructions. In another instructional, we learn how to draw resonance constructions of nitrate ion.
Total choice of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anionThere are one nitrogen atom and 3 oxygen atoms in the nitrate ion. Also there is a -1 charge on the nitrate ion.
Nitrogen and oxygen are situated at VA and VIA teams respectively within the periodic desk. So nitrogen has 5 electrons in its valence shell. In oxygen atom, there are six electrons in its valence shell.
Total valence electrons given by way of nitrogen atom = 5There are three oxygen atoms in NO3-, Therefore
Total valence electrons given via oxygen atoms = 6 *3 = 18Due to -1 price, some other electrons is added
Due to -1 charge, gained electrons to valence electrons= 1 Total valence electrons = 5 + 18 + 1 = 24Total valence electrons pairsTotal valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are made up our minds through dividing the number total valence electrons through two. For, NO2-, there are 24 valence electrons, so total pairs of electrons are 12.
Center atom of NO2-To be the center atom, ability of getting higher valance is important. Nitrogen can display valence,5. But, oxygen's most valence is 2. Therefore nitrogen has the extra likelihood to be the center atom (See the determine). So, now we can construct a sketch of NO3- ion.
Sketch of NO2- ion Lone pairs on atomsThere are already three N-O bonds within the comic strip. Therefore simplest nine valence electrons pairs are remaining to draw the remainder of ion.
Start to mark those nine valence electrons pairs on out of doors atoms (oxygen atoms) as lone pairs. One oxygen atom will take three lone pairs following the octal rule (oxygen and nitrogen atoms can not keep greater than 8 electrons in their valence shells). All nine valence electrons pairs (9) are spent when lone pairs are marked on oxygen atoms.
Therefore, there is not any ine valence electrons pairs to mark on nitrogen atom.
Check the stableness of drawn NO2- ion and minimize charges on atoms by converting lone pairs to bondsCheck fees on atoms and mark them as below. Charges are important to decide the lewis structure of the ion.
The drawn structure for NO3- is not a solid structure because oxygen atoms and nitrogen atoms have charges. When a molecule or ion has so many charges on atoms, that structure isn't solid.
Now, we should attempt to decrease fees by converting lone pair or pairs which exist on oxygen atoms to bonds. So we convert one lone pair of one oxygen atom as a N-O bond.
Now there's a double bond between nitrogen and one oxygen atom. There are also two unmarried bonds (N-O) with nitrogen atom and different oxygen atoms.
In new structure, fees of atoms are diminished. Now there is not any any fee on one oxygen atom. Also, rate of nitrogen atom is decreased from +2 to +1. Now you realize this structure of NO3- is extra solid than earlier structure due to much less charges on atoms.
But, We cannot convert more lone pairs of alternative oxygen atom to make a bond with nitrogen atom because nitrogen can not stay greater than 8 electrons in its ultimate valence shell.
Questions
Ask your chemistry questions and find the answers How many lone pairs are across the nitrogen atom in nitrate ion?No electrons pairs exist on nitrogen atom. But, on nitrogen atom, there's a +1 charge. Around the nitrogen atom, there are two unmarried bonds and double bond.
Related tutorialsEC PS4 - Problem Set 4 - CHEM 230 - StuDocu

56 Chapter 5: Electron Configuration, Lewis Dot Structure, And Molecular Shape Ele
Untitled
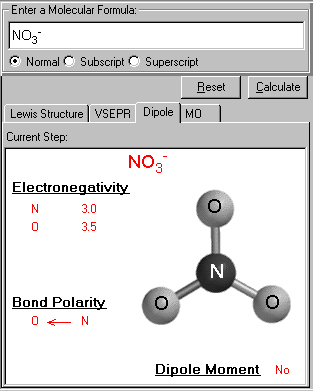
Bond Polarity And Dipole Moment
CHEMISTRY 103 – Practice Problems #3 Chapters 8 – 10 Http://www.chem.wisc.edu/areas/clc (Resource Page) Prepared By Dr. Tony
Name______________________________________

Lewis Structure And Resonance
A Level GCE Shapes Of Molecules Appendix Shapes Of Oxyanions KS5 AS A2 Revision Notes

There Are 3 Lewis Structures For [ NO3]- D... | Clutch Prep
![There Are 3 Lewis Structures For [ NO3]- D... | Clutch Prep There Are 3 Lewis Structures For [ NO3]- D... | Clutch Prep](https://i0.wp.com/lightcat-files.s3.amazonaws.com/clutch_answers_images/1570068268116.jpg)
Wich Of The Following Are Isoelectonic And Isostructural No3 Co3 2 So3 Clo3 A No3 Co3 2 B So3 No3 C Clo3 Co3 2 D Co3 2 So3 Only One Correct And
O-d 6,.- Cs '1
7.4 Formal Charges And Resonance – Chemistry

Ch3cno Lewis Structure - Fill Online, Printable, Fillable, Blank | PdfFiller

Solved] Consider The Precipitation Reaction. Pb(NO3)2( Aq ) + 2 LiCl( Aq ) PbCl2( S ) + 2 LiNO3( Aq ) Write The Reaction Showing The Lewis Structure... | Course Hero
Name _____Mr. Perfect_____________________________ Date ____Sp 15________ Chemistry 101 Quiz 6 1. Write Lewis Structures For T
Solved: Please Do Number 17. Draw The Lewis Structures Of | Chegg.com

Lewis Structures

Chemistry Net: Method For Lewis Dot Structures: The Nitrate Ion Lewis Structures

Chemistry 105, Chapter 7 Exercises

What Is The NH4 Lewis Structure? - Quora
Hybridization Of Oxygen In Nitrate Ion And The Location/bond Of Nitrogen's Lone Pair - Chemistry Stack Exchange

0 Comment to "CCl4 Lewis Structure | Science Trends"
Post a Comment